Navigating Multi-Dose Vials: A 2025 Information to 28-Day Expiration Calendars and Protected Medicine Administration
Associated Articles: Navigating Multi-Dose Vials: A 2025 Information to 28-Day Expiration Calendars and Protected Medicine Administration
Introduction
With nice pleasure, we are going to discover the intriguing subject associated to Navigating Multi-Dose Vials: A 2025 Information to 28-Day Expiration Calendars and Protected Medicine Administration. Let’s weave attention-grabbing info and supply contemporary views to the readers.
Desk of Content material
Navigating Multi-Dose Vials: A 2025 Information to 28-Day Expiration Calendars and Protected Medicine Administration

Multi-dose vials (MDVs) are a standard function in healthcare settings, providing comfort and cost-effectiveness for administering drugs requiring a number of doses. Nevertheless, their use necessitates meticulous monitoring of expiration dates to make sure affected person security and stop medicine wastage. This text offers a complete information to managing MDVs, focusing particularly on the implementation of 28-day expiration calendars for 2025 and past. We’ll discover greatest practices, potential challenges, and technological options to optimize MDV administration.
Understanding Multi-Dose Vials and Their Expiration:
MDVs comprise a bigger amount of medicine than single-dose vials, permitting for a number of withdrawals over a time period. Crucially, as soon as an MDV is opened, the medicine’s sterility and efficiency are compromised. This necessitates the implementation of strict expiration insurance policies to attenuate the danger of an infection and drugs degradation. Not like unopened vials which adhere to the producer’s said expiration date, opened MDVs have a considerably shorter shelf life, sometimes restricted to twenty-eight days post-first use.
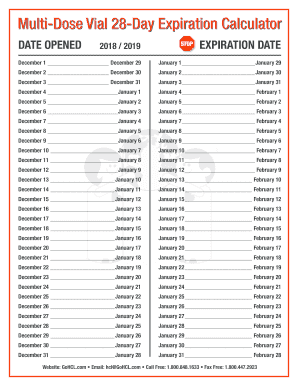
The Significance of 28-Day Expiration Calendars:
A 28-day expiration calendar is a crucial software for managing opened MDVs. It offers a transparent, visible report of when the vial was first accessed and when it should be discarded. This method ensures that:
- Affected person Security: Medicine stays potent and free from contamination, decreasing the danger of hostile reactions or therapy failures.
- Medicine Waste Discount: By adhering to the 28-day restrict, healthcare suppliers forestall the pointless disposal of probably usable medicine.
- Compliance and Accountability: A documented system enhances compliance with regulatory tips and offers a transparent audit path for medicine administration.
- Improved Effectivity: Clear labeling and monitoring techniques streamline workflows, saving time and sources for healthcare professionals.
Implementing a 28-Day Expiration Calendar System for 2025:
Efficiently implementing a 28-day expiration calendar system requires a multi-faceted strategy:
-
Clear Labeling: Every opened MDV should be clearly labeled with the date of first use. This may be finished utilizing a everlasting marker, a pre-printed label, or a specialised labeling system. The label must also embrace the medicine identify, focus, and the 28-day expiration date (calculated from the date of first use).
-
Designated Storage: Opened MDVs needs to be saved in a chosen space, separate from unopened vials, to forestall unintentional misuse or contamination. Correct storage circumstances, as specified by the producer, should be maintained (e.g., refrigeration).
-
Calendar System: A bodily calendar or a digital system can be utilized to trace the 28-day expiration dates of all opened MDVs. This method needs to be readily accessible to all healthcare professionals concerned in medicine administration. For 2025, a devoted calendar particularly designed for this function needs to be used, both printed or digitally maintained.
-
Employees Coaching: Complete coaching for all healthcare personnel concerned in dealing with MDVs is essential. This coaching ought to cowl correct labeling methods, storage procedures, calculation of expiration dates, and the significance of adhering to the 28-day restrict.
-
Common Audits: Periodic audits needs to be performed to make sure compliance with established protocols. This consists of checking the accuracy of labeling, the correct storage of MDVs, and the adherence to the 28-day expiration dates.
Addressing Potential Challenges:
Implementing and sustaining a strong MDV administration system presents a number of potential challenges:
- Human Error: Mislabeling, inaccurate date calculations, or overlooking expiration dates are widespread human errors that may compromise affected person security. Strong coaching and common audits are essential to mitigate this threat.
- Workflow Integration: Integrating the 28-day expiration calendar system into current workflows requires cautious planning and coordination to keep away from disrupting current practices.
- Know-how Integration: Whereas expertise can improve MDV administration, its implementation requires cautious consideration of compatibility, value, and coaching wants.
- Employees Turnover: Excessive employees turnover can result in inconsistencies in MDV administration if correct coaching and documentation are usually not maintained.
Technological Options for Enhanced MDV Administration:
Know-how provides a number of options to beat these challenges and improve MDV administration:
- Barcode Scanning: Integrating barcode scanning into the medicine administration course of can automate knowledge entry and scale back the danger of guide errors.
- Digital Medicine Administration Data (eMARs): eMARs can present a centralized, digital report of all opened MDVs, their expiration dates, and drugs administration occasions.
- Automated Meting out Cupboards (ADCs): ADCs can monitor MDV utilization and alert healthcare professionals when a vial is nearing its expiration date.
- Devoted Software program: Specialised software program options can be found to handle MDV stock, monitor expiration dates, and generate experiences to observe compliance.
Conclusion:
Efficient administration of multi-dose vials is paramount for affected person security and environment friendly useful resource utilization. The implementation of a strong 28-day expiration calendar system, significantly for 2025 and past, is essential. This includes a mixture of clear labeling, designated storage, employees coaching, common audits, and the strategic integration of expertise. By addressing potential challenges and leveraging technological developments, healthcare suppliers can create a secure and environment friendly system for managing MDVs, making certain optimum affected person care and minimizing medicine waste. The dedication to those practices is not going to solely enhance affected person outcomes but in addition contribute to a extra sustainable and cost-effective healthcare system. The yr 2025 and past ought to see a major enchancment in MDV administration on account of a larger emphasis on affected person security and the adoption of improved applied sciences. Common evaluate and adaptation of those techniques are important to keep up their effectiveness and adapt to evolving healthcare wants.





Closure
Thus, we hope this text has supplied useful insights into Navigating Multi-Dose Vials: A 2025 Information to 28-Day Expiration Calendars and Protected Medicine Administration. We admire your consideration to our article. See you in our subsequent article!